The history of mankind is full of mysteries and riddles, some of which have already been solved, but many have yet to be understood and deciphered. The mystery of the Baghdad Battery falls into the second category...
In 1936, during archaeological excavations at Quyut Rabbua, near Baghdad, a strange object was found that dates back to the Parth civilisation that existed in the second century B.C. The object consists of a yellow clay shell in the shape of an elongated vase, the size of a palm. Inside the vessel, held by a lid, is a small copper cylinder 9 cm long and 26 mm wide, also, inside it, also held by a lid, is an iron rod. Wilhelm König, then director of the Iraqi Museum in Baghdad, noted similarities with papyrus containers found in Seleucia. Its outward resemblance to a carbon-zinc battery (common "lantern" batteries) led Koenig to speculate that it might be a galvanic generator.
It is not easy to prove or disprove the hypothesis that this is indeed a battery. Indeed, this object, like any other composed of two different metals, can function as an elementary battery if immersed in an acid solution. In tests, however, it turns out that the current generated is minimal. It is not easy to get electricity of a reasonable strength and make a battery work for more than a few minutes if only two metals, copper and iron, are used and if no modern acids, which were hardly known at the time... Although.... this again, is just a guess. Based on the fact that what is known to modern scientists was not available to the inventors of the past.
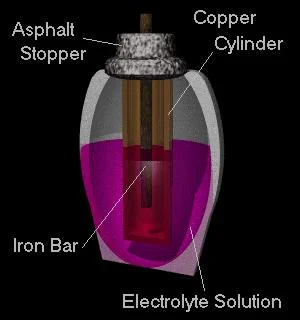
In a battery, electricity is generated by two different reactions occurring near the two electrodes, between them and certain substances (electrolytes) dissolved in the liquid in which they are immersed. Different types of electrolytes have been suggested, based on substances known at the time of the 'battery''s creation. If acidified or salted water is used, it only acts as a conductor, allowing the reactions to take place: Fe-> Fe2+ + 2 e- O2 + 2 H2O + 4 e- -> 4 OH-
The second reaction takes place with oxygen dissolved in the water. In this case, therefore, the sealed form of the 'stack' is an unfortunate choice, as the necessary oxygen is difficult to dissolve in the water, a metal grid placed directly below the surface in the pool would have worked much better. As the object found by König was a sealed cylinder, it could only work for a few minutes, more promising candidates for a 'prehistoric battery' are similar objects found in Seleucia.
Gray tried using copper sulphate and it worked, but again only for a short time before the iron electrode became coated with copper. Jansen et al. used benzoquinone, a substance found in the secretions of some millipedes, mixed with vinegar. All of these processes work very poorly because the Baghdad battery lacks a mechanism (such as a porous septum or gelatin) that separates the electrolytes reacting with the two electrodes. Nevertheless, there is a remote possibility that this object was indeed an elemental battery, and it is not beyond the technical capabilities of the time.
You can try making a 'Baghdad battery' at home. All you need is a piece of iron, some electrical waste, a glass of vinegar (or copper sulphate solution) and an electronic tester for amateurs. Connect the piece of iron to the wire and immerse it in the solution. A second wire, stripped to a length of a few centimetres, can be used as the copper electrode. You will be able to see for yourself that although the voltage produced can reach one volt, the current is very small, no more than a few milliamps. You can also have fun experimenting with all sorts of substances as electrolytes.

The mystery of the Baghdad battery - unmasking
According to proponents of the theory, the battery could be used to produce gold plating or even galvanic objects. If a single battery does not produce sufficient current or voltage, it is sufficient to put several in parallel or in series. But we have no archaeological evidence of galvanised objects and no known artefacts show that they were gilded using galvanic techniques.
In support of this hypothesis, Koenig cites the fact that among artisans in Baghdad today the technique of electroplating is used, in which the object to be gilded is immersed in a solution of cyanide gold salts in a porous vessel immersed, in turn, in a salt solution. The necessary current is produced by the oxidation of a piece of zinc immersed in salt water and electrically connected with the object to be gilded. This method, however, is very similar to the process patented last century in England, of which it is probably an adaptation, and contains important differences from the "pile": zinc, much more easily oxidised than iron, a porous partition between the two electrolytes, the use of cyanide salts, unknown at the time.
On the other hand, there are many clues that lead one to think of this object as a container for sacred scrolls used for magical or propitiatory purposes. Various metals were used to represent deities and there are parallels with similar containers used for this purpose. No metal wires or other indications of its "electrical" use were found near it, in particular no wire was found, as depicted in most depictions of this object, which would have been necessary to electrically connect the copper cylinder.
But even if the "battery" hypothesis is correct, if the Persians did create a rudimentary generator, it would have been just another one of those promising discoveries that were lost to time and no one ever realised their enormous potential. There is no need to invoke the mysterious influence of the Atlanteans or aliens: if they really wanted to teach our ancestors how to create batteries, they could have provided a less primitive and inefficient method than this.


 and then
and then 
